Heavy water- Structure and application
We know that water (H2O) contains two hydrogen atoms and one oxygen atom. Heavy water, on the other hand, is water in which both hydrogen atoms have been replaced with deuterium. Its chemical formula is D2O and is present naturally in water, but in only small amounts approximately one water molecule per twenty million water molecules.
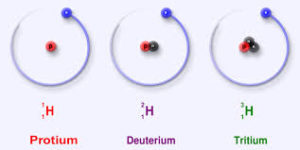
Hydrogen has three isotopes- protium, deuterium and tritium. Deuterium is an isotope containing one proton and one neutron. Thus heavy water is water that contains heavy hydrogen or deuterium. The most common isotope of hydrogen is protium, which has one proton and no neutrons. Deuterium differs from the hydrogen usually found in water, protium, in that each atom of deuterium contains a proton and a neutron. Because deuterium contains a neutron, it is more massive or heavier than protium and so is it named ‘heavy hydrogen’. It is also less volatile than H2O.Heavy water may be deuterium oxide, D2O or it may be deuterium protium oxide, DHO.
Heavy water is widely used to run nuclear reactors. Heavy water is used as a moderator of neutrons which allow a nuclear reactor to operate with natural uranium as its fuel. Role of the moderator is to slow down the neutrons released by nuclear fission so they have more time to react with the nuclear fuel. Deuterium is used as a tracer in nuclear fusion reactors and to slow down neutrons in heavy water moderated fission reactors. It is also used as a tracer in the study of living organisms, chemical and bio chemical processes.
An adult human body naturally contains deuterium in very small amounts. Larger concentration of heavy water (25-50%) in some species is considered toxic.









This post is invaluable. How can I find out more?
Awesome article.
As the admin of this website is working, no uncertainty very rapidly it will be renowned, due to its quality contents.