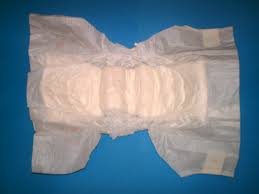
Diaper: How does it work?
When water is poured on a dry diaper, it absorbs them and the top layer remains dry. This is because of the presence of a super-absorbent layer found in diapers. Now, the magic of absorbing lot of water is done by a chemical present in the super-absorbent layer. It is Sodium polyacrylate.
Sodium polyacrylate is a white, granular solid. It is a polymer composed of chains of identical monomers –CH2—CH(CO2Na)–. It is a sodium salt of polyacrylic acid. The water changes into solid crystals when poured over a layer of sodium polyacrylate keeping the upper layer dry. Sodium polyacrylate can absorb as much as 200-300 times its weight in tap water, 800 times its own in distilled water and 30 times its own weight in the case of urine. The water absorption is said to happen through the process of osmosis. When in contact with water, in order to bring about equilibrium both inside and outside the superabsorbent layer, sodium atoms move from the crystals into the water. When these atoms leave, they are replaced with water.
It is non toxic but could cause nasal irritation or irritation in the eyes, lungs if inhaled. It has a wide variety of commercial and industrial uses. It is used as a chelating agent in many detergents in order to bind the elements (present in hard water), dirt and other substance found in water as well as clothes, making the detergent more effective in cleaning. It is used as a thickening agent in hair gels as it absorbs water and changes them into crystals. It also protects the optical and electrical cables from moisture. In gardening, it is added to potted plants and soils in order to retain moisture. It acts as a water reservoir soaking up excess water and discharging when necessary. An addition of teaspoon of salt (sodium chloride or calcium chloride) change the crystals back to water.