How are elements classified?« Back to Questions List
|
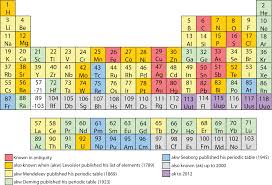
Initially all the elements were only classified into two groups namely metals and non metals. The main disadvantage was that the metals group was quite big and certain elements had the properties of both metals and non metals. Hence there was a need for a more lucid classification. Russian chemist Dmitry Mendeleev arranged the elements based on their chemical and physical properties in a tabular form known as the ‘periodic table’. It is ‘periodic’ in the sense that after a certain interval, the physical and chemical properties repeat themselves. He derived this table based on the theory that the “physical and chemical properties of an element are a periodic function of their atomic masses”. He arranged the elements according to the increasing order of their atomic masses in a row and after an interval the element that possess the same properties as the first was placed in the second row. Though the first periodic table was a historic achievement, it had few drawbacks. On further study, the modern periodic table was discovered by an English scientist Henry Moseley. He found that the fundamental property of an element is the atomic number and not it’s mass. Atomic number is the number of protons in the nucleus of an atom. The modern periodic law states that “physical and chemical properties of an element are a periodic function of their atomic numbers”. The periodic table based on this modern periodic law is called the modern periodic table. Many forms of this modern periodic table are used but the one commonly used is called the long form of periodic table. Horizontal rows are called periods and the vertical columns are called groups.
|